Data Management
Archival of Images
Multi-site architecture that enables smooth management of images and reports across multi geographical sites.
A foolproof Disaster Recovery Plan that includes policies, process, and procedures to ensure business continuity in case of any disaster to ensure the resumption of critical functions in the event of any unscheduled interruption.
ICL’s powerful archival solution offers seamless data integration and built-in analytics that gives you more powerful control over your data. Now all your imaging reports are available without the need to download any special software.

Generation of EDC
We can assure you a unique depth of experience and capabilities for Diagnostic Imaging in Clinical Trials.

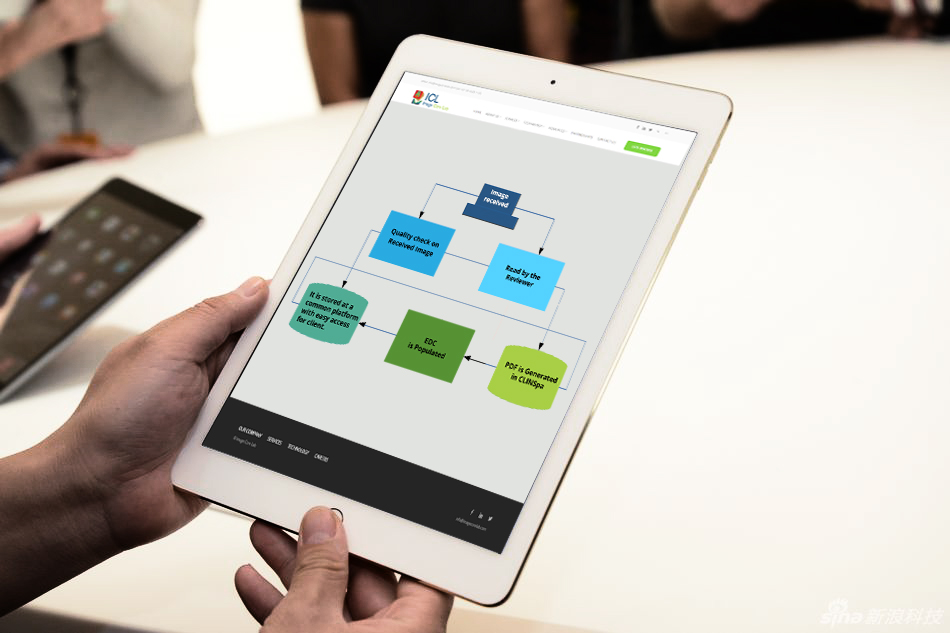
Image received

Quality check on Received Image

Read by the Reviewer

PDF is Generated in CLINSpa

EDC is Populated

It is centrally stored for easy client access
Integrated Image management Solutions for every phase of your clinical trial that will optimize your costs & offer best in class Image Core Labs solutions. Be it in any Phase (Phase I, Phase II, Phase III or Phase IV) of clinical trial of pharmaceutical, biotechnology or medical device product, we are one stop solution for all your imaging needs.
