The outbreak of Covid-19 disease has dramatically altered clinical trials. The US Food and Drug Administration (FDA) released guidelines during the pandemic to promote clinical trial execution remotely viz-a-viz safeguarding patients and preserving acceptable clinical practice standards. Decentralized trials (DCTs), described as trials conducted via telemedicine, mobile/local healthcare providers (HCPs), and/or mobile technologies, are not constrained by the geographic constraints that plague traditional trials, providing enhanced accessibility to the patients. It paves the way for the stakeholders like physicians or researchers to have better insights into real-world data collection, product compliance and efficacy, and patient behaviors.
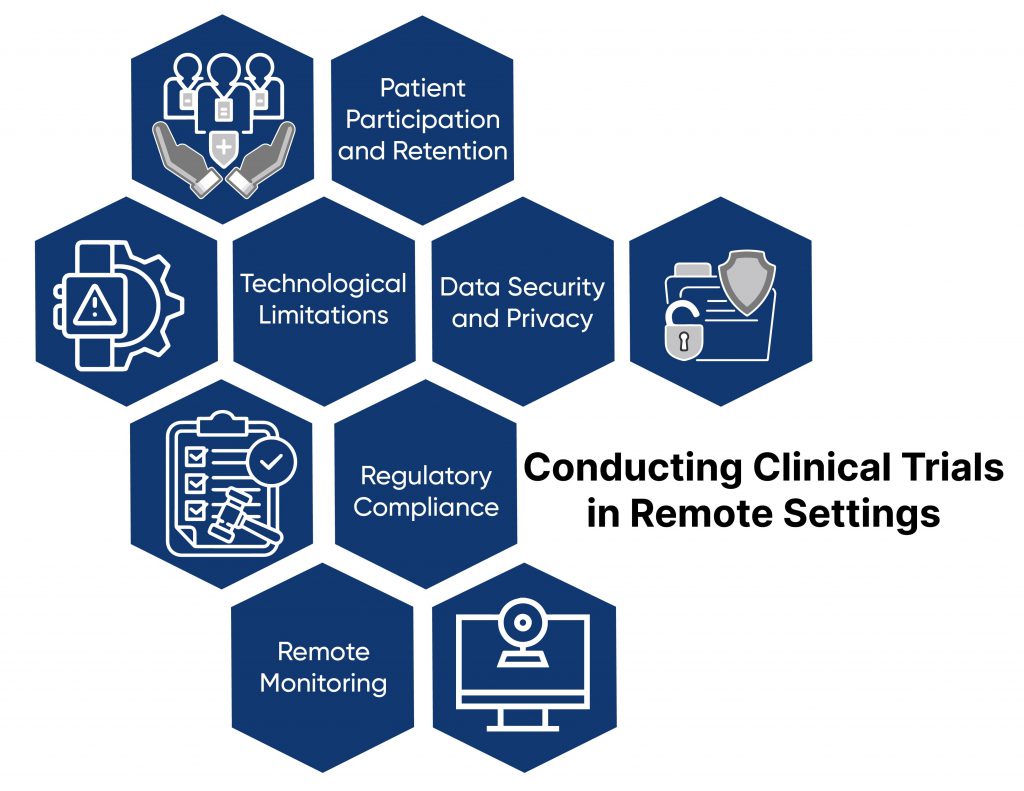
1. Patient Participation and Retention:
Traditionally, participants or patients physically visit research centers or sites to participate in clinical trials. Maintaining engagement and retention is challenging for remote trials. Mobile apps, wearable devices, and virtual check-ins can enhance personalized, interactive engagement.
2. Patient Participation and Retention:
Traditionally, participants or patients physically visit research centers or sites to participate in clinical trials. Maintaining engagement and retention is challenging for remote trials. Mobile apps, wearable devices, and virtual check-ins can enhance personalized, interactive engagement.
3. Technological Limitations:
Not all participants have equal access to technology or digital literacy, impacting inclusivity. Technological assistance from community centers with user-friendly interfaces and training resources can help overcome this challenge.
4. Data Security and Privacy:
Electronic platforms for communication and data collection raise concerns about patient information security. Data safety monitoring procedures are crucial to ensure compliance and protect patient privacy.
5. Regulatory Compliance:
Adhering to regulations like HIPAA is complex in remote settings. Establishing proactive regulatory strategies, early contact with authorities, and staying updated on regulations are essential for compliance.
6. Remote Monitoring:
Monitoring clinical trial data remotely using digital technology presents challenges in ensuring data quality and integrity. Reliable remote monitoring systems with AI-based predictive analytics improve data oversight efficiency.
To summarise, despite presenting several challenges, remote clinical trials possess enormous potential for innovations and improved patient outcomes. Researchers could harness the untapped potential of remote settings by addressing these issues head-on with strategic tactics and leveraging technologies. Keeping adaptive with the ever-evolving industry and embracing novel solutions will be critical to the success of remote clinical trials.
Image Core Lab is a turnkey solution for every phase of the remote clinical trial of any pharmaceutical, biotechnology, or medical device product. We are steadfast in making the world a healthier place for everyone.