Study design- An important aspect of a clinical trial
Study design is an important aspect of the clinical trial. A good study design defines the aim, purposes, motives and plans of the trial within the practical constraints of location, time, money and availability of staff. It helps in proving a hypothesis. The hypothesis provides the justification for the clinical trial. Just as the key elements and determinants of a military campaign are fixed even before it is begun, the endpoints and expected outcomes of a clinical trial are also set at its initial planning stage.
Every clinical trial begins with the development of a clinical protocol that is compliant with regulatory/GCP requirements. A trial protocol describes how a clinical trial will be conducted, its background, rationale, objectives, design, methodology, statistical considerations etc.
Study designs are based on the type of study that’s being conducted; and can be experimental and observational. There are two fundamental cornerstones that a study design needs to comply with for a successful output-Reliability and Validity. Reliability of study design is determined if the outcome of the trial is replicable. It refers to the reproducibility of the measurements/ outcomes of a trial when repeated at random on the same subject or specimen. Validity refers to the ability of a study/method to accurately measure what it intends to. These two factors dictate sound study design as they help prevent any form of bias.
Designing of a study involves multiple steps. A study design decides the right randomization techniques which have the potential to reduce the selection bias. Apart from randomization, it determines the inclusion and the exclusion criteria; endpoints and how those endpoints will be measured, i.e. determining the biomarker.
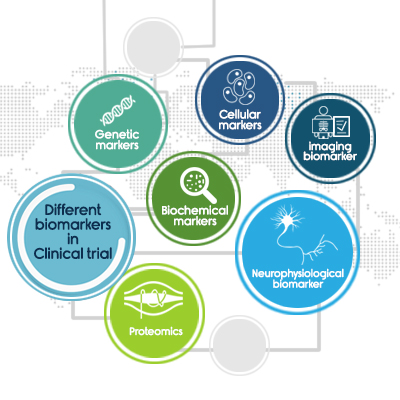
Different biomarkers in Clinical trial
A biomarker is a term derived from the term “Biological Marker”. National Institutes of Health defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. A biospecimen biomarker is derived from patient tissue or biofluids (Urine, blood etc.). They are the quantifiable characteristics of biological processes.
There are different types of markers such as biochemical markers, cellular markers, cytokine markers, genetic markers, physiological results, imaging biomarker, neurophysiological biomarker, and proteomics (protein-based biomarkers).
Biomarkers are further classified as target biomarkers (does the drug hit its target and, therefore, provide benefit) and efficacy biomarkers (indicators of positive drug effect via the mechanism of drug action).
Choosing a disease-specific biomarker aids in a more rapid and accurate disease diagnosis; potential reduction in size and duration of the trial, which would in turn speed up the trial submission.
Imaging Biomarker
An imaging biomarker is a biological characteristic that is detectable on an image. Imaging biomarkers are the cornerstone of modern radiology, and imaging endpoints are a critical necessity for patient safety and understanding a drug’s effectiveness. They are key to making appropriate therapeutic decisions and drug evaluations which is why they require advanced multidisciplinary expertise to provide the most accurate information possible.
Imaging Biomarker can be
1. Anatomical or Functional
Anatomical image biomarkers refers to the longest diameter of a lesion, size etc. while functional imaging biomarkers refer to a physiological aspect of a tumour in the image such as oxygenation levels, cellularity or vascularity.
2. Qualitative or Quantitative
Qualitative Imaging biomarker is descriptive while quantitative imaging biomarker refers to an objectively measured parameter such as the longest diameter of the nodule decreased by 5mm after treatment as compared with its size before treatment.
Imaging Biomarkers in Oncology Trials
Imaging Biomarkers are integral to the routine management of patients with cancer. An imaging biomarker is a measurement derived from one or more medical images. Imaging studies can be readily acquired, and are cost-effective, non-invasive tools for screening and detection of tumours. They are also used for serial monitoring of patients, including assessments of response to therapy and identification of therapeutic complications. Imaging biomarkers enable tracking of a particular tumour or a lesion repeatedly over time and can also map the morphologic characteristics of tumours, and at the same time can evaluate multiple different lesions independently within an individual.
An imaging biomarker is frequently used in clinical TNM stage, objective response and left ventricular ejection fraction etc. CT, MRI, PET and ultrasonography biomarkers are used extensively in cancer research and drug development.
Several different imaging Biomarkers can rely on different response criteria. For Instance, RECIST criteria (Response Evaluation Criteria in Solid Tumor) relies on the measurement of lesion size as a determinant of tumor response to the therapeutic agent.
Prognostic and predictive IB
A prognostic Imaging biomarker provides information about the patient’s overall cancer outcome, regardless of the therapy.
TNM staging system records the presence, size and the number of lesions at a tumour, nodal and other metastatic sites. The American Joint Committee on Cancer (AJCC) has set detailed radiology reporting guidelines for cancer staging enabling measurements to be accurately reproduced. For solid tumours, TNM staging is used and the images acquired are typically CT, MRI, single-photon emission computed tomography and PET which may or may be supported with biospecimen or clinical measurements.
However, in some cases, TNM is also used as a predictive Imaging biomarker. In prostate cancer, clinical TNM stage distinguishes localized disease from the locally advanced disease. In this scenario, the imaging biomarker (IB) is prognostic because individuals in the former group have worse outcomes than those in the latter group. The same IB, however, can be used as an indicator of response to therapy.
Imaging Biomarker in Neurological Trials
Imaging biomarkers play an important role in determining the rate of disease progression in case of Alzheimer’s disease (AD). This allows better tracking of changes over a period of pathoanatomic or pathophysiologic alterations in the brain as seen in cases of the AD. With the help of imaging-based measures or imaging biomarkers, it also may be possible to detect the ability of a potentialdisease-modifying agent to impede the degenerative process of AD.
Some imaging-based biomarkers have begun to show preliminary promise as potential markers of AD traits or presymptomatic or prodromal disease states. Longitudinal studies of individuals at elevated genetic risk and of subjects with mild memory impairment suggest that it may be possible to use imaging measures to identify “leveraged cohorts” of individuals who are at elevated risk for a clinical diagnosis of AD within several years.
Imaging biomarker in Musculoskeletal trails
Radiological endpoints in clinical trials for the evaluation of the musculoskeletal system are numerous and have a unique set of challenges that are usually disease specific. The imaging modalities employed for these endpoints include conventional radiography, computed tomography, ultrasound, dual X-ray absorptiometry, bone scintigraphy and magnetic resonance imaging; and the measurement criteria used are Van Der Heijde Modified Sharp Score; RAMRIS Scoring; Fracture Healing assessment.
Imaging Biomarkers in pharmaceutical industry – Challenges and Solutions
Regardless of the therapeutic area, the phase of trial or indication, adding imaging as a part of a trial further complicates it by new regulatory and workflow compliance challenges. A few of those are:
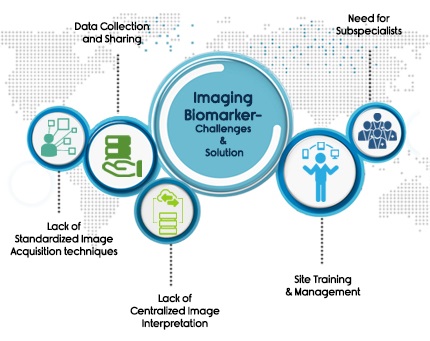
1) Site Training & Site Management
2) Need for subspecialist
3) Data collection and sharing
4) Lack of Standardized Image Acquisition techniques
5) Lack of Centralized Image Interpretation
Choosing the right core lab can be the one-stop solution for all these hurdles.
At Image Core Lab we understand the unique challenges that pharmaceutical companies face when it comes to adding imaging in clinical trials.
We aim to leverage our deep set expertise in technology and image interpretation service to accelerate drug development trials at the right speed.
To find out more about our Services, or how we can help you manage imaging requirements of your trial talk to us today.